SG-Nasal test
A Test For The Early Diagnosis Of Nasal Cancers
Head-neck neoplasms can benefit from epigenetics
Epidemiology of nasal cancer
Sinonasal cancers are rare entities comprising 5% of all cancers of the head and neck region, with an annual incidence of about 1 case per 100,000 population worldwide, accounting for 0.5-1% of all malignancies in the Western population. Sinonasal squamous cell carcinoma (SNSCC) and intestinal-type adenocarcinoma (ITAC) account for 80% of all sinonasal cancers. They occur most commonly in adult and elderly men, with a male:female ratio of 2:1 in SNSCC and up to 6:1 in ITAC.
The male predominance of sinonasal tumors is probably the result of the etiologic involvement of occupational hazards. The oncogenesis of sinonasal tumors has been related to occupational exposure to several industrial compounds, the most frequent of which is wood and leather dust, in about 40% of all cases, 30% of SNSCCs and 90% of ITACs specifically. Exposure to such substances present especially in the work environment usually begins at a young age and often persists for more than 20 years. Workers in the wood industry have a risk up to 500-900 times and 20 times higher of developing ITAC and SNSCC, respectively, than the general population. In addition to wood and leather dust, chemicals such as glues, formaldehyde, chromium, nickel and various compounds used in the textile industry have been associated with sinonasal carcinomas, especially SNSCC. Based on this evidence, ITAC is officially considered an occupational disease in many European countries.
Clinical need
The symptoms of nasal cavity cancer share the same clinical presentation as many other more common lesions, such as chronic rhinitis or sinusitis. Because of the nonspecificity and often relatively mild nature of early symptoms, malignant tumors of the nasal cavities have a prolonged diagnostic latency, which often leads to the discovery of these tumors at an advanced stage.
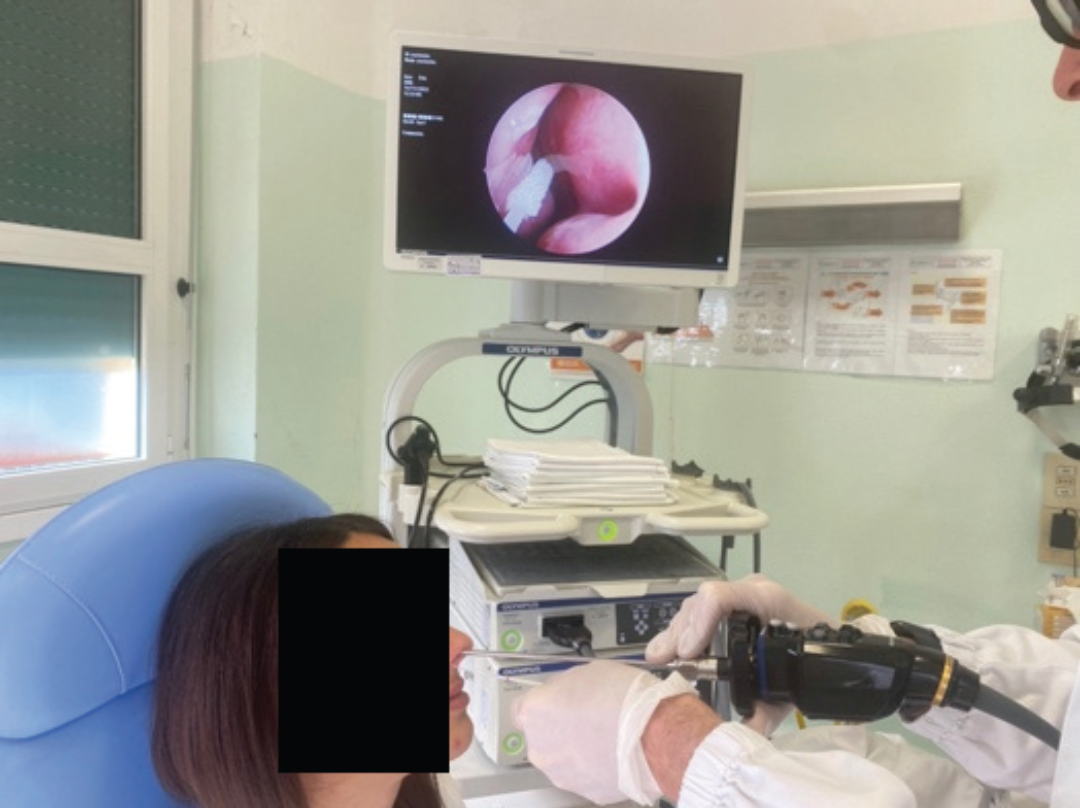
Early diagnostic tools and appropriate preoperative diagnosis are needed to detect patients with neoplastic lesions in the early stages. Diagnosis of a sinonasal tumor requires nasal endoscopy and biopsy sampling, procedures that can create discomfort and may be refused by the patient, lengthening the time to identify these carcinomas.
These procedures are also necessary for the diagnosis of sinonasal tumors.
Therefore, the development of non-invasive methods for early detection could be the winning strategy to reduce the burden of nasal cavity carcinoma and could be useful for medical surveillance of workers at risk.
The Technology
Studium Genetics has developed and patented a method for early, non-invasive detection of patients with sinonasal carcinoma. The innovation is based on epigenetic analysis (assessment of DNA methylation) by parallel sequencing (NGS) of 13 genes. The starting material is cells taken from the sinonasal mucosa with a flexible flocked swab, making the test completely non-invasive. The markers involved in this invention are 13 genes whose modifications at the level of DNA methylation have been widely demonstrated in all head-neck cancers. The analysis of their methylation patterns allows for the calculation of a score to identify patients with sinonasal cancer. The test has shown an analytical sensitivity of 83.78% and a specificity of 94.7%.
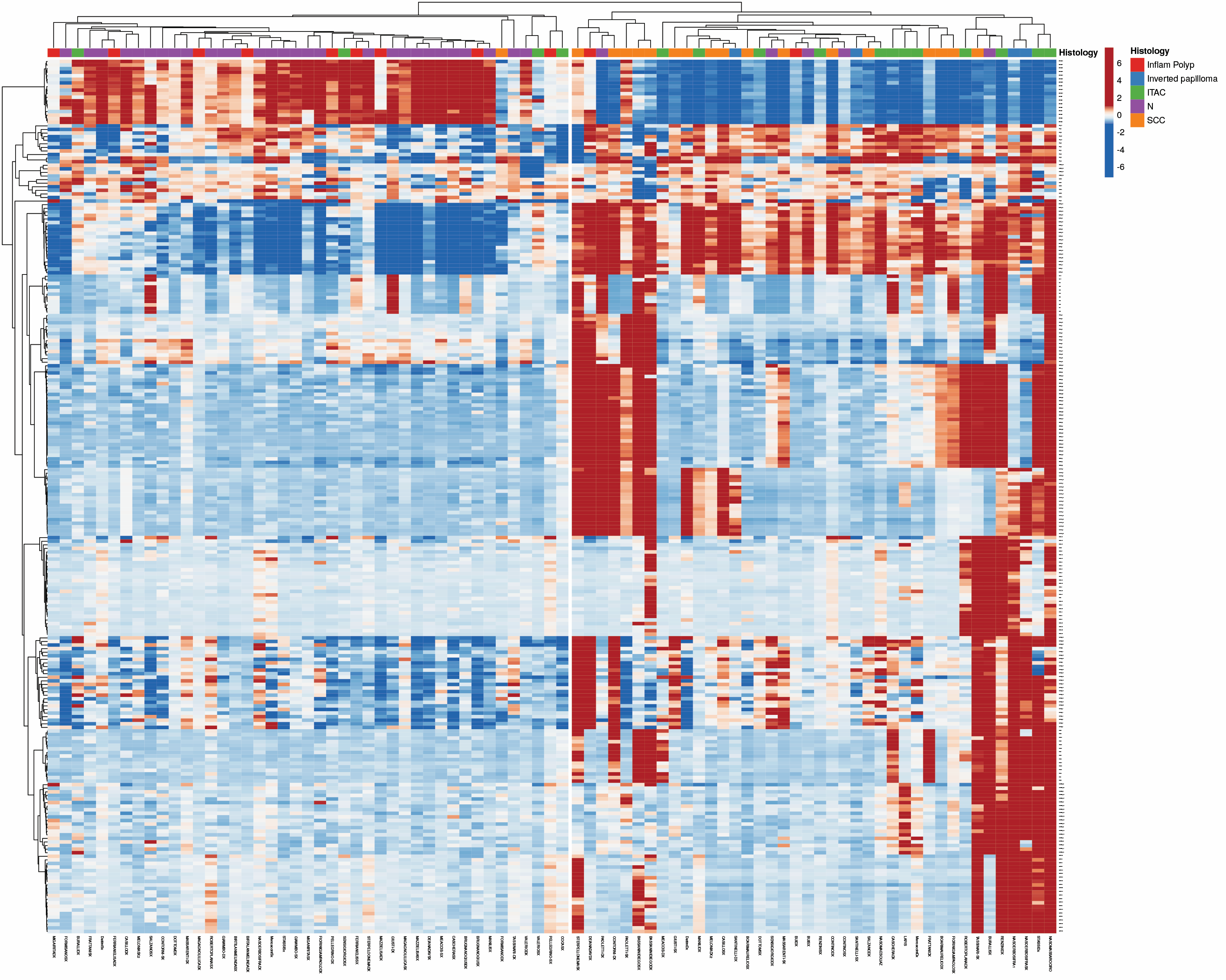
The chart below shows the ability to stratify by methylation pattern for the various groups examined: healthy donors (in purple), inflammatory polyps (in red), inverted papillomas (in blue), ITACs (in green), and carcinomas (in orange).
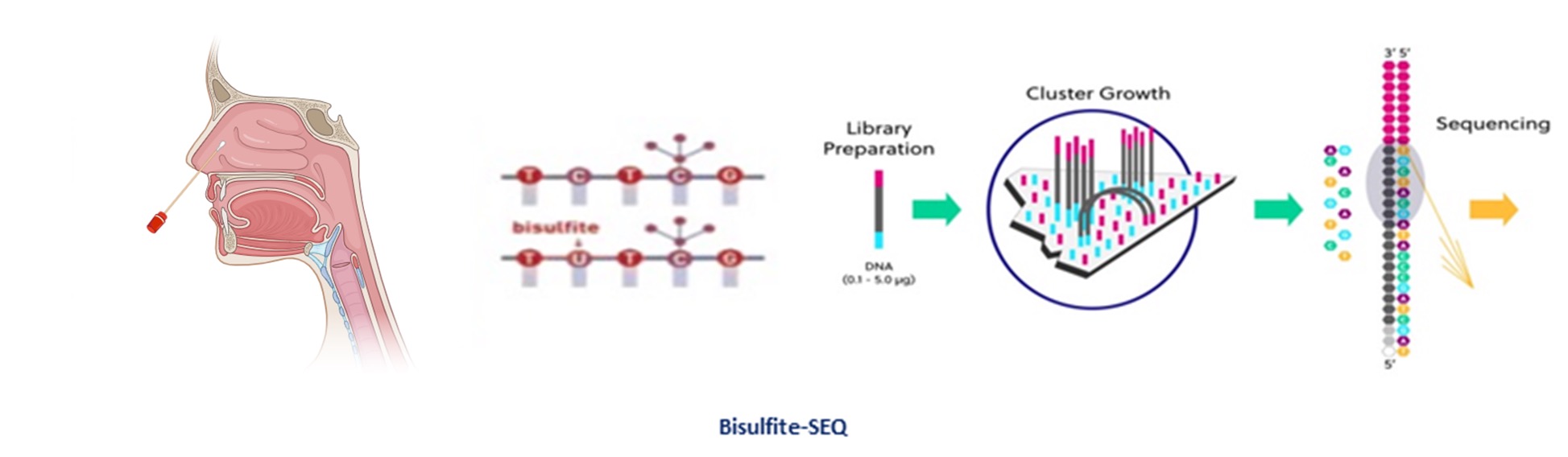
Test execution
Nasal mucosa sample collection with flocked swab and sample stabilization with preservation solution. The sample can be stored at room temperature for up to one month to allow shipment without cold storage. Once the sample arrives at the centralized NGS laboratory, it will be processed by parallel sequencing after treatment with sodium bisulfite (NGS) for quantitative evaluation of 13 genes. Through a proprietary patented algorithm, a score will be calculated from the data obtained: if it exceeds the threshold of 1.06 it will be considered positive and thus associated with the presence of sinonasal carcinoma.
Uniqueness of the test
High sensitivity without being invasive.
Based on Next generation Sequencing methods with high precision sodium bisulfite.
The sample can be delivered at room temperature.
Indicates tumors at an early stage, improving the patient's chance of survival and reducing the invasiveness of surgery, with positive consequences for recovery and postoperative quality of life.
Quick sample collection protocol, ideal for second-level screening programs.
There are currently no alternative technologies on the market for the early detection of sinonasal carcinoma. Although several approaches have been tried in the past, none of them have been validated on clinical specimens and implemented in clinical practice.