Oral Cancer
Oral squamous cell carcinoma (OSCC) is a major public health challenge with more than 745,000 cases reported worldwide by IARC. Despite treatment modalities including surgery, radiotherapy and chemotherapy, the 5-year mortality rate remains approximately 60%. In addition, the development of a second primary OSCC after surgery ranges from 17% to 30% and is higher than any other tumor type and is the leading cause of cancer-related death.
In current clinical practice, oral cancer screening is performed solely by visual inspection by general practitioners or dentists. In cases of suspicious lesions, patients are referred to oral and maxillofacial surgeons for further diagnostic biopsy. However, biopsy requires a minimally invasive surgical approach that can cause discomfort and may be refused by the patient, reducing the ability to detect these cases. In addition, early detection may be hindered by the clinical experience of the clinician and the subtle nature of the lesions, which may not be detectable by oral examination alone.
Currently, there is no commercially available method for the early detection of oral cancer. In recent decades, several non-invasive diagnostic tools have been proposed, including toluidine blue staining, autofluorescence, chemiluminescence, exfoliative cytology, and optical diagnostic methods such as Raman spectroscopy, elastic scattering, diffuse reflectance, narrow band imaging, and fluorescence visualization. However, these methods have several limitations related to low sensitivity and specificity, and therefore their clinical applications have not been developed.
Epidemiology of Oral Squamous Cell Carcinoma
Oral and pharyngeal cancer, grouped together, are the sixth most common cancer in the world, and it's prevalence is increasing. The annual estimated incidence is approximately 750,000 per year, two-thirds of these cases occurring in developing countries (source: International Agency for Research and Cancer 2023).
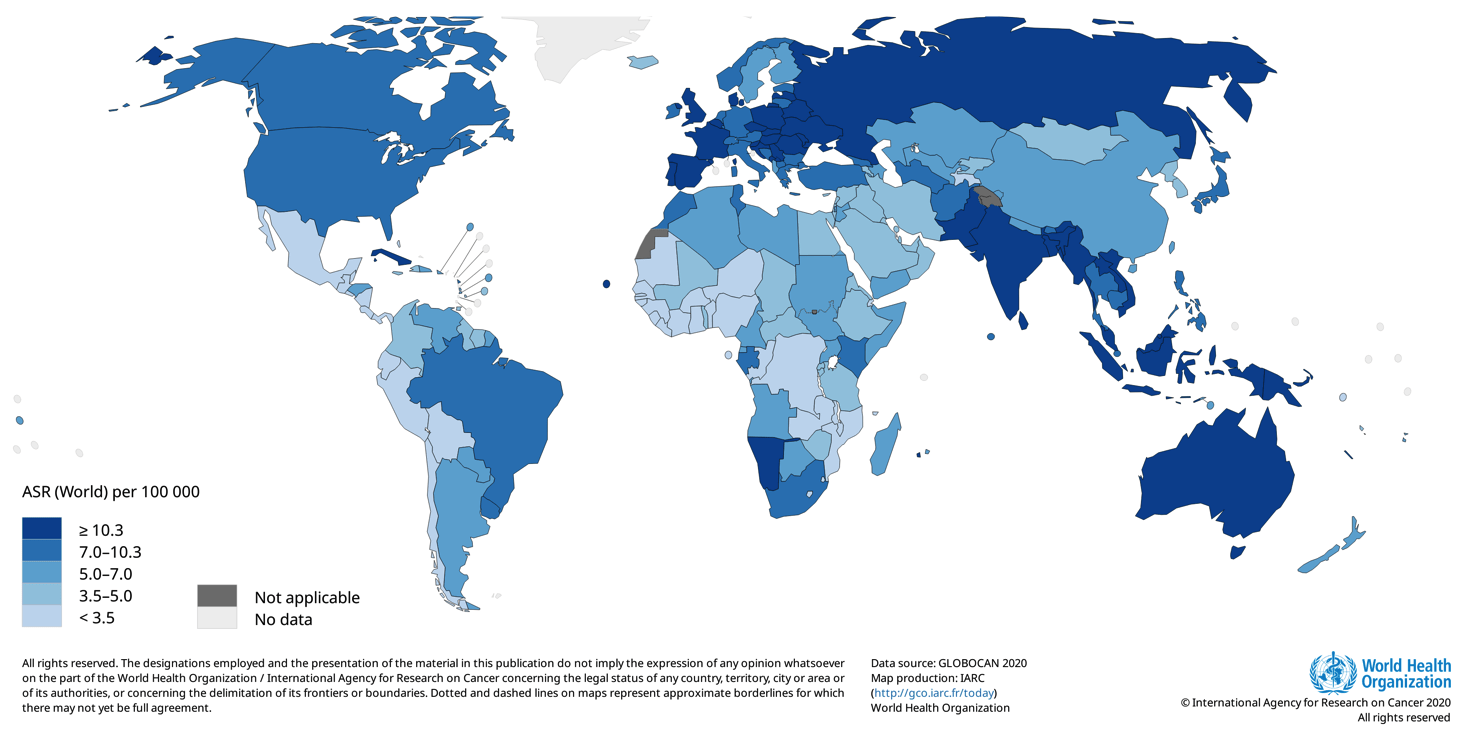
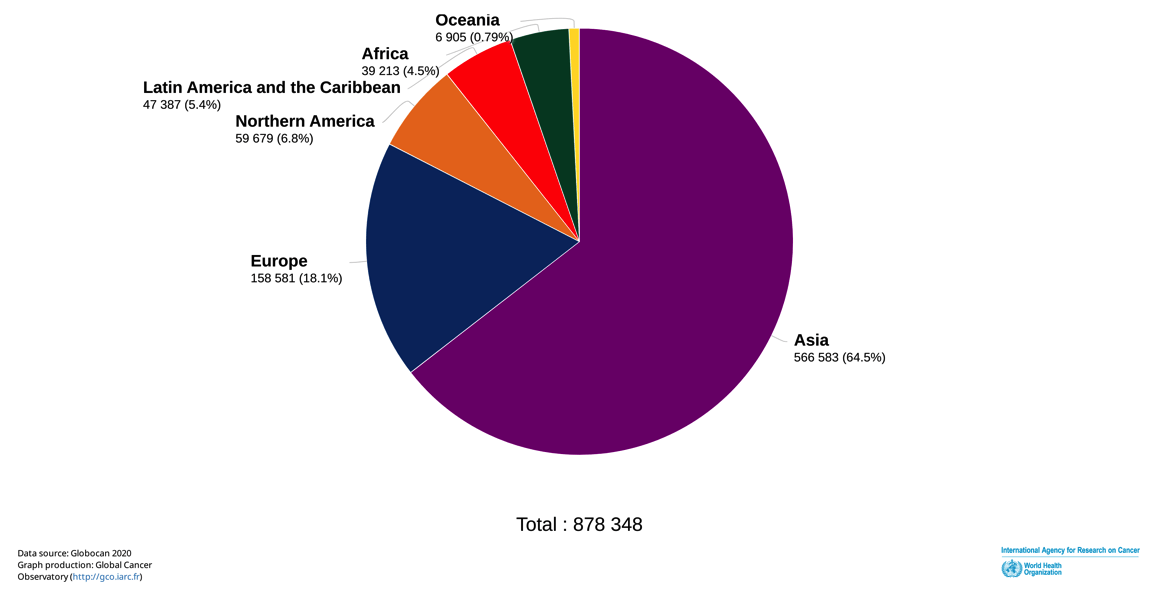
The estimated number of new cases per year considering lip and oral cavity, larynx, hypopharynx, nasopharynx, oropharynx for each continent are as follows: Europe: 158,581 (Italy: 9,300); Asia: 566,583; Northern America: 59,679; Latin America and the Caribbean: 47,387; Africa: 39,213, Oceania: 6,905.
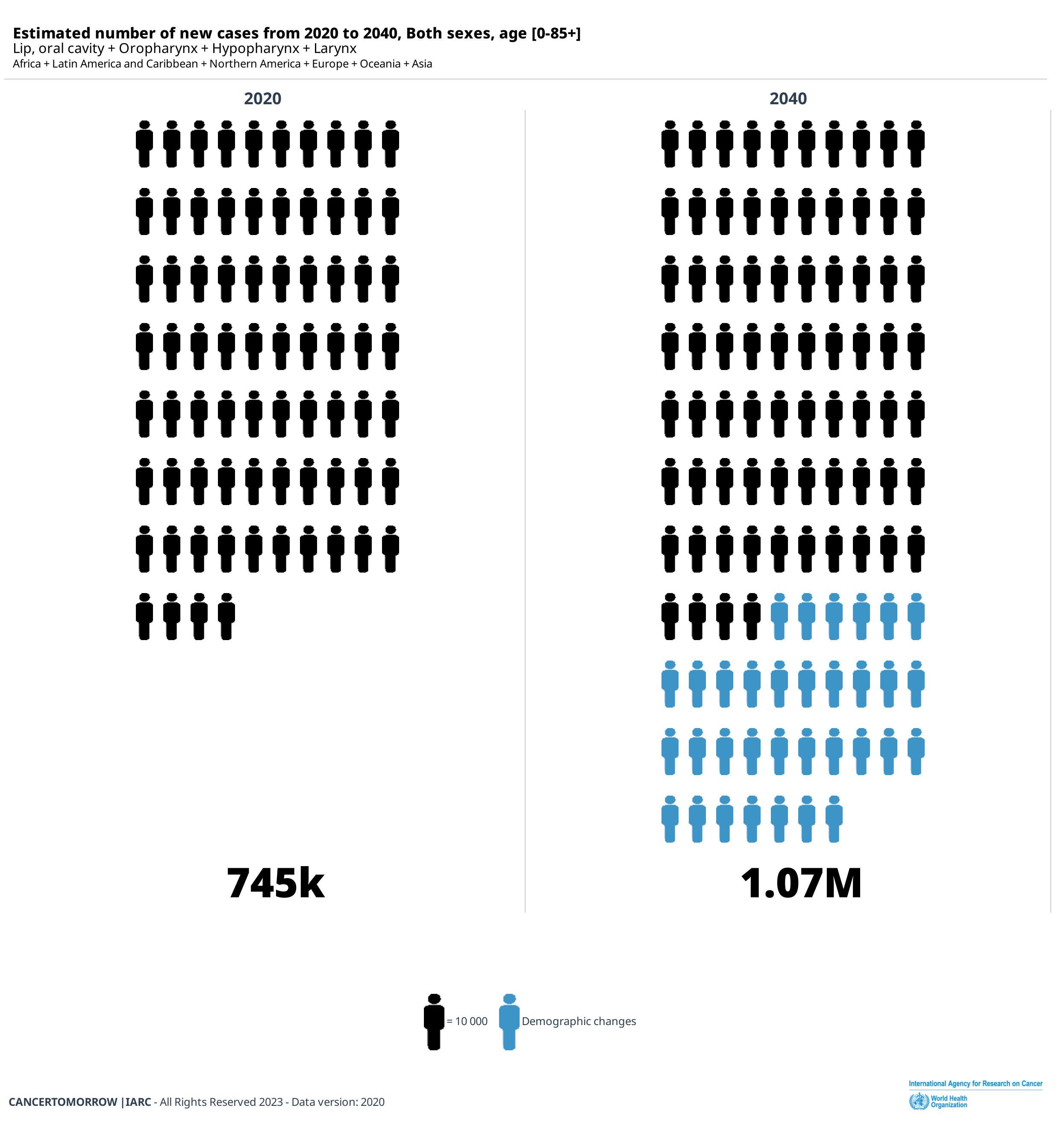
The overall incidence of Head and Neck Squamous Cell Carcinoma (HNSCC) continues to rise, with a predicted 30% increase annually by 2030 (Sung H et al. CA Cancer J Clin 2021; 71: 209–249), including developed and developing countries. The increasing incidence in Europe and USA has been attributed to a rise in oropharyngeal cancer related to human papillomavirus (HPV) infection; in Asia, this is due to population growth and the association to chewing of areca nuts (betel quid) and tobacco leaves. Overall, HNSCC affects men two to four times more than women, with estimates reaching over 20/100,000. In developing countries, lip and oral cavity carcinoma also known as oral squamous cell carcinoma (OSCC), which represents approximately 90% of HNSCC, is the second most common cancer (Miranda-Fihlo A et al. Oral Oncology 2020). The risk of HNSCC increases with age with the majority of cases diagnosed after the fifth decade of life.
Risk Factors
Tobacco smoking and alcohol intake are well established risk factors for oral cancer. There is a strong dose-response relationship for the risk of oral cavity and oropharyngeal cancer, with increasing frequency and duration of both smoking and alcohol consumption. Smoking duration is more important than frequency for oral cancer, with fewer cigarettes per day over a longer number of years having a higher level of risk for oral cancer. Betel quid with or without tobacco commonly used in South and Southeast Asia was shown to have a near three-fold increased risk association. Oral human papillomavirus (HPV) is mainly associated with oropharyngeal (rather than oral cavity) cancer risk. Oral HPV is sexually transmitted and a recent multi-center study found HPV positive oropharyngeal cancer case proportions of 60% in US and 31% in Europe. Low fruit, vegetables, and consequently low beta-carotene and vitamin-C intake have been associated with an increased risk of oral cancers. Lack of dental care and not following regular oral hygiene practices may cause an increased risk of oral cancer. Poor dental health or ongoing irritation from poorly fitting dentures, especially in people who use alcohol and tobacco products, may contribute to an increased risk of oral and oropharyngeal cancer.
Limits of the current diagnostic procedures
Despite surgery, radiotherapy, and chemotherapy treatment modalities, the 5-year mortality rate remains approximately 60%. Furthermore, the development of a second primary OSCC after surgery ranged between 17% and 30%, and it is higher than any other type of tumor being the leading cause of cancer-related death.
Although the oral cavity is easily accessible for examination, only about 30% of oral cancers are detected at an early stage (I and II) and before metastasis to other sites, which are reported in 40% of cases.
In current clinical practice, oral cancer screening is performed solely by oral visual examination by general physicians or dentists. In cases of suspicious lesions, patients are referred to oral medicine specialists or maxillofacial surgeons for further diagnostic biopsy. However, the biopsy requires a minimally invasive surgical approach that can create discomfort and may be refused by the patient, reducing the power of intercepting those cases. Moreover, the early detection might be hindered by practitioners' clinical experience and the subtle nature of the lesions, which may be undetectable by oral examination alone.
Currently, no method allowing for oral cancer early detection is commercially available. In the past few decades, several noninvasive diagnostic tools have been proposed, including toluidine blue staining, autofluorescence, chemiluminescence, exfoliative cytology, and optical diagnostic method, such as Raman spectroscopy, elastic scattering, diffuse reflectance, narrow-band imaging, and fluorescence visualization. However, these methods have several limitations related to low sensitivity and specificity, and this is why their clinical applications were not developed.
High risk patients are smokers and alcoholics, and above all patients with Oral Premalignant Lesions (OPML) detected by dentists, maxillofacial surgeons, otolaryngologists such as leukoplakia (white plaque), erythroplakia (red plaque) and oral lichen planus (OLP, an inflammatory disease), as defined by WHO. Leukoplakia is defined as a white patch or plaque that cannot be characterized clinically or pathologically as any other disease. It has a comprehensive global review point at a prevalence of 2.6% and malignancy conversion rate ranging from 3.5% to 5%. For erythroplakia, 75–90% of lesions prove to be carcinoma, carcinoma in situ, or are severely dysplastic. OLP is the consequence of a chronic cell-mediated immune condition of unknown etiology. The reported annual malignant transformation rate for OLP is about 0.5%.
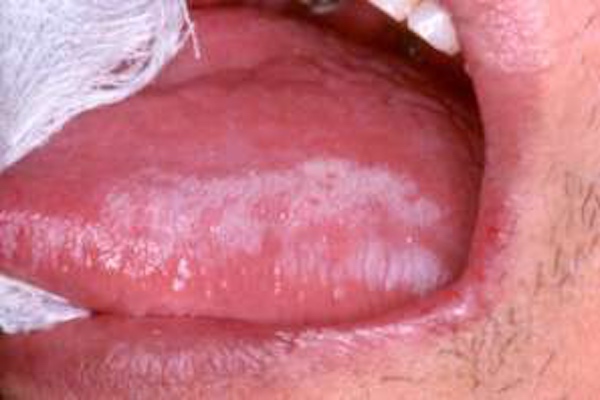
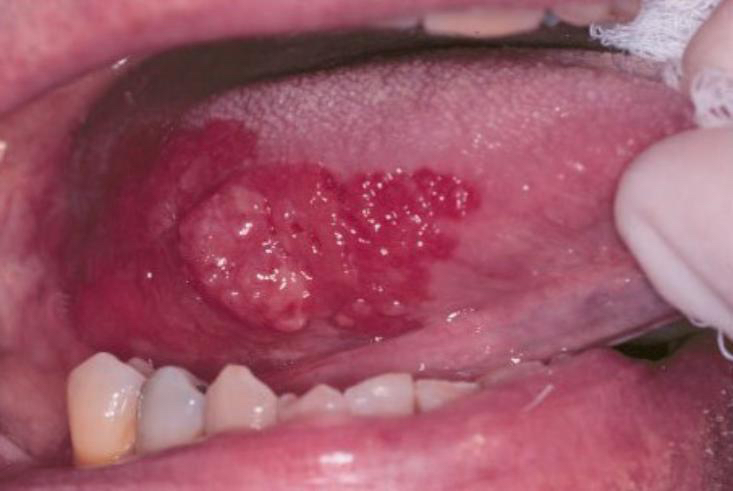
Clinical and histological features of OPML are not able to provide enough information to identify the lesions, leading to a high risk of enduring malignant transformation and develop an OSCC during follow-up. Although the oral cavity is easily accessible for examination, several factors limit the identification and early treatment of OPMLs. For this purpose, the current gold standard for screening and detecting, is the visual and tactile palpation during an extra- and intra-oral examination by the healthcare professional in a routine dental or physical examination.
However, this disease is not easy to identify in its earliest stages and has often eluded medical and dental professionals because it can be “occult,” or hidden from plain view. Indeed, normal-looking tissue may often hide the truth within the cells below the mucosa’s surface.
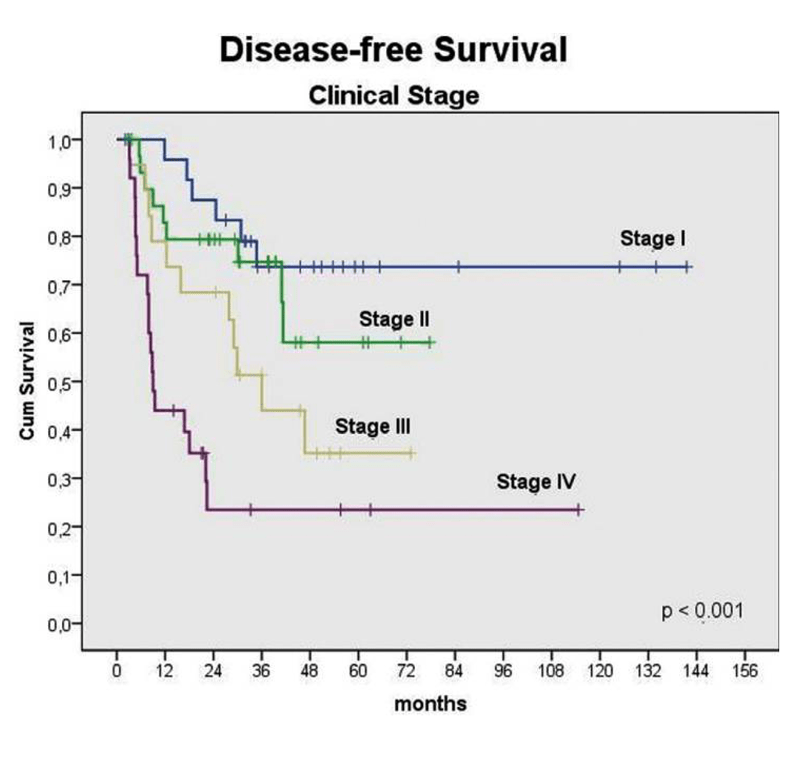
If the disease is identified in earlier stages (Stage I or Stage II) - the ideal time for identification is before the dysplastic cells have been able to break through the basement membrane - the overall five-year survival rate is greater than 80 percent.
Unfortunately, all too often the manifestations of this invasive and devastating disease are detected in the late stages (Stages III-IV), when the lesions have typically advanced so deeply that it is impossible to treat without radical surgical intervention and significant loss of the patient’s quality of life.
In particular, OSCC is usually diagnosed based on an incisional biopsy. Nevertheless, the incisional biopsy requires a minimally invasive surgical approach that can create discomfort and be refused by the patient, inevitably delaying the diagnosis and further treatment options.
It’s clear that an early diagnosis will decrease the mortality rate and also the cost of the health care system or the insurance company in some Countries. If diagnosed in stage I and II, patients will be treated in day hospital, giving the opportunity to go back to work in a few days with a very limited risk of relapse during follow-up. This allows them to support their families in a reduced time, limiting the socio-economic costs.
Table 1: the cost of HNSCC treatment in relation to stage in selected countries.
| Article | Country | Data | Cost per patient |
| EUROPE | |||
| Polesel et al. (2019) | Italy | Health system cost (Capital cost + recurrent cost) database | Stage I: 12,969 Euro Stage II: 18,276 Euro Stage III: 26,229 Euro Stage IV: 25,574 Euro |
| St Guily et al. (2010) | France | Hospital cost oral cavity cancer patients | Public: 7,673 Euro Private: 3,054 Euro |
| Zavras et al. (2002) | Greece | Diagnosis, inpatient hospitalization period and length, clinical stage, operating room visits, radiation therapy, chemotherapy, reconstruction, complications, secondary operating procedures and ICU days | Stage I : 3,662 USD Stage II: 5,867 USD Stage III: 10,316 USD Stage IV: 11,467 USD Mean: 7,450 USD |
| Van Agthoven et al. (2001) | Netherlands | Diagnosis, treatment and follow-up (max. 2 years) of primary tumours. | Mean: 25,425 Euro |
| WORLD | |||
| Yang et al. (2021) | China | Hospitalization cost | Median: 10,297.90 USD |
| Raman et al. (2021) | Malasya | Outpatient care, biopsy, investigation, inpatient care | Early stage: MYR 56,820; (11,932 Euro) Late stage: MYR 71,536 (15,147.7 Euro) |
| Huang (2020) | Taiwan | Direct costs in the first 3 years after diagnosis | 19,644 USD |
| Amarasinghe et al. (2019) | Sri Lanka | Health system cost (Capital cost + recurrent cost) | Stage II: 394 USD Stage III and IV: 2,024 USD |
| Pollaers et al. (2019) | Australia | 1-year overall cost 2-year overall cost | 1-year overall cost: Stage I: 54,917 USD Stage II: 107,861 USD Stage III: 101,010 USD Stage IV: 127,844 USD 2-year overall cost: Stage I: 51,208 USD Stage II: 95,682 USD Stage III: 120,129 USD Stage IV: 133,817 USD |
| Lee et al 2010 | Taiwan | Hospitalization cost | Mean: 9,528 USD |
WHO encourages developed Countries to prioritize basic, high-impact and low-cost early diagnosis of cancer. Early diagnostic approaches greatly reduce cancer’s financial impact, because the cost of treatment is much less in early stages, and people go back to work in a few days. In 2010, the annual economic cost of cancer through healthcare expenditure and loss of productivity was estimated at USD 1.16 trillion.
